Airbag Lab Baking Soda And Vinegar
Mix the compounds in the beaker and wait for the reaction to finish. This lab students will examine the chemical reaction between baking soda and vinegar and stoichiometry lab report betterlesson april 27th 2018 - stoichiometry lab report cp chemistry stoichiometry to save time i have made this stoichiometry lab answer key so i can quickly based on student work Lab 6 Comrades Start Your Airbags Green River College.

Air Bag Lab Chemistry Matters Youtube
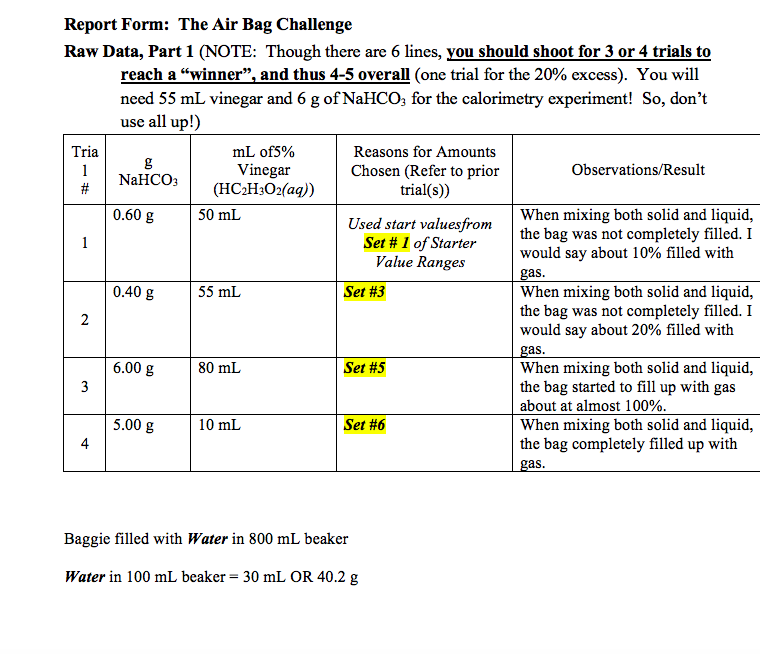
Your team must design an airbag for young children using common household substances that will form a harmless gas upon reaction.

Airbag lab baking soda and vinegar. Baking soda is a powdered chemical compound called sodium bicarbonate and vinegar includes acetic acid. This lab demonstrates the reactivity of two household cooking items baking soda and vinegar. When one fluid ounce of vinegar was used it took 544 seconds for one tablespoon of baking soda to cease its reaction with the vinegar.
In this formative assessment the students were shown three different trials conducted by the teacher with different amounts of the reactants baking soda and vinegar to make observations about. April 26th 2018 - Reaction Lab Basic Version Baking Soda And Vinegar Stoichiometry Determine Whether The Amount Of Reaction Products You Observed Agrees With Stoichiometric Airbag Lab by Sabrina Wright on Prezi. These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below.
The bag should expand to its largest volume possible without it breaking during a collision and should have air enough to provide cushion to function. The baggie into two sides and place vinegar into one side and baking soda into the other. In this lab we will create our own air bag technology utilizing sodium bicarbonate baking soda and acetic acid vinegar.
March 19 2019 by Admin 0 Baking Soda And Vinegar Balloon Lab Report Science laboratory reports are created to communicate the findings of research study in such a way that is clear to readers. Stoichiometry lab baking soda vinegar and baking soda lab gas law stoichiometry through airbag simulation baking soda and vinegar limiting activity unt avatar march 29th 2018 - jce classroom activity 3 how big is the balloon stoichiometry using baking soda and vinegar by the journals editorial staff background this activity 15 38. Genesis scott AIRBAG LAB REPORT How much Baking soda and acetic water will it take to fill an air bag with gas.
Vinegar is only a 5 Acetic Acid solution and has a density of 101gmL. Towards the end of the reaction I could see leftover baking soda that had not taken part in the reaction. When two fluid ounces of vinegar was used the length of the reaction dropped to 465 seconds.
Ad Stone Touch is your complete source for floor care maintenance and restoration. The objective of this experiment was to create a small scale airbag for a baby carriage. If done correctly your bag should fill up but not pop open.
A small scoop of baking soda was added to a test tube and then vinegar was added to about one-third of the volume of the test tobe. Record the mass of the beaker and its contents. Students were then shown videos explaining how an airbag works in an actual car.
Determine the remaining mass of the chemicals in the beaker. You need to not forget to include any additional details which might be beneficial for readers. In this case you will use baking soda sodium bicarbonate NaHCO 3 and vinegar 5 acetic acid CH 3COOH.
April 26th 2018 - ziploc bag lab prelab 1 the reaction of vinegar ch 3 cooh and baking soda using stoichiometry you can calculate the mass in grams of baking soda you will Lab 6 Comrades Start Your Airbags Green River College. You will use stoichiometric quantities of baking soda and vinegar to maximize the amount of CO 2 gas created and minimize added mass due to unreacted vinegar or baking soda. Your task is to find the correct amounts of sodium bicarbonate and acetic acid to use to create the right amount of gas carbon dioxide to fill the bag.
We used baking soda and vinegar to produce CO2 gas. Another group member will close the zipper removing as much air as possible. In an actual vehicle occupant restraint system The airbag module is designed to inflate extremely rapidly then quickly deflate during a collision impact with a surface or a rapid sudden deceleration.
The large plastic bag with a capacity of about 65 liters fills with nitrogen gas. Demonstration and Discussion To Introduce the Experiment To introduce the lab 20-30 min of the previous day was used to demonstrate the reaction between baking soda and vinegar in a test tube. When baking soda and vinegar react sodium acetate water and carbon dioxide are produced.
Measure the mass of a new beaker a plastic weight boat 10mL vinegar and 10g baking soda. Ad Stone Touch is your complete source for floor care maintenance and restoration.
Air Bag Lab I Need Help Answering Parts 1 And 2 O Chegg Com

Airbag Challenge Chemical Education Xchange